1 in the dianion of 4-hydroxybenzoic acid to 1650 cm. Spectra for six anions from 1700 to 1250 cm 1 are compared with experimental data.
A CO stretch falls at about 1700 1 and a C-O stretch typically falls at about 1200 5.
. The frequency of the carboxylate stretching vibration near 1550 cm. Theoretically then a C-O bond and a half stretch might fall at the average peak position of these two carbonoxygen stretching vibrations. Why is the carbonyl IR stretch in an ester higher than in a ketone.
Infra-red spectroscopy of molecules was introduced 110 years ago by Coblentz as the first functional group spectroscopic method The structure of the compound has a great influence on the absorption spectra. Use of a continuum solvent model improves over calculations performed in vacuum. A linear standard curve using anhydrous sodium salicylate has been obtained from the plot of carboxylate COO concentration vs.
1780 - 1710 s 1690 - 1630 s The carbonyl stretching absorption is one of the strongest IR absorptions and is very useful in structure determination as one can determine both the number of carbonyl groups assuming peaks do not overlap but also an estimation of which types. Sutton Gabriel da Silva and George V. The weight of the IR band area using the COO antisymmetric stretch vibration at 1580 cm 1.
The average of 1700 and 1200 is 1450 close to where the carboxylate stretching peaks fall. In comparison with its corresponding free ion a large splitting of the co 2 stretching frequencies δ ν is often an indication of monodentate coordination in a metal carboxylate. A combined experimental and theoretical study of carboxylate.
You could also have an asymmetric stretch like this. 3700 - 3500 m. University of Central Missouri.
The variations of the frequency differences of symmetric and asymmetric stretching vibrations in a series of carboxylato FeII complexes have been theoretically studied. So if these hydrogens are both stretching at the same time thats a symmetric stretch. It is shown that structural information can be obtained from a direct comparison between the difference Delta nuas - nus.
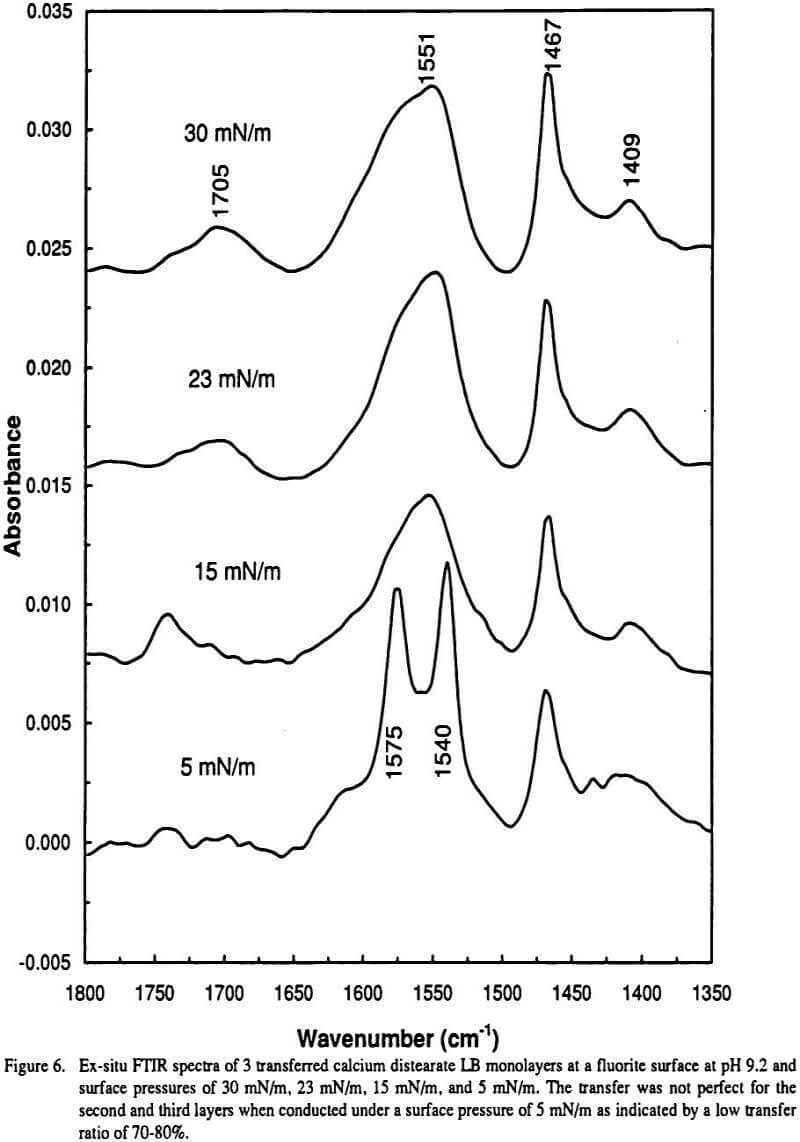
The effect of molecular conformation is examined and is found to be minor. In many cases it seems as though certain bonds are due to certain. On the other hand the doublet IR absorbance band at 1540 and 1575 cm1 which is observed for adsorbed carboxylate groups at multilayer coverage and for bulk-precipitated calcium dicarboxylate.
Modelling the IR spectra of aqueous metal carboxylate complexes. And once again youll find the asymmetric stretch so this one actually takes a little bit more energy. Spectra were recorded in regions below 800 cm 1 8001500 cm 1 the fingerprint region the region between 2800 and 3000 cm 1 C-H stretch region and finally the region between 3000 and 3600 cm 1 O-H stretch region by method as mentioned by Belton et al.
We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. IR carboxylate stretching modes are widely used to infer if the geometry of the bonding is monodentate or bidentate. 24 After deposition of R 12 C the ATR-IR data show CH stretching and bending bands of the alkyl chain 25 along with asymmetric and symmetric carboxylate stretching bands for the linker group at 1540 and 1398 cm 1 respectively 26-28.
The asymmetric stretching frequency increases and the symmetric one decreases due to the breakdown in equality of the carbonyl group mehrotra and bohra 1983 colthup. Infrared spectra are calculated for aqueous carboxylate ions using DFT. The surface composition and morphology of the TiO 2 coated HDG-steel substrate have been in detail previously.
You can have a symmetric stretch and an asymmetric stretch. Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate. Weatherability of pastel and dark colored rigid PVC compound can be achieved by substituting the more light.
We report the first IR spectroscopic observation of carboxylate stretching modes in free. Correlation between bonding geometry and stretching mode wavenumber shifts Catherine. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal centre can be used to infer if the.
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
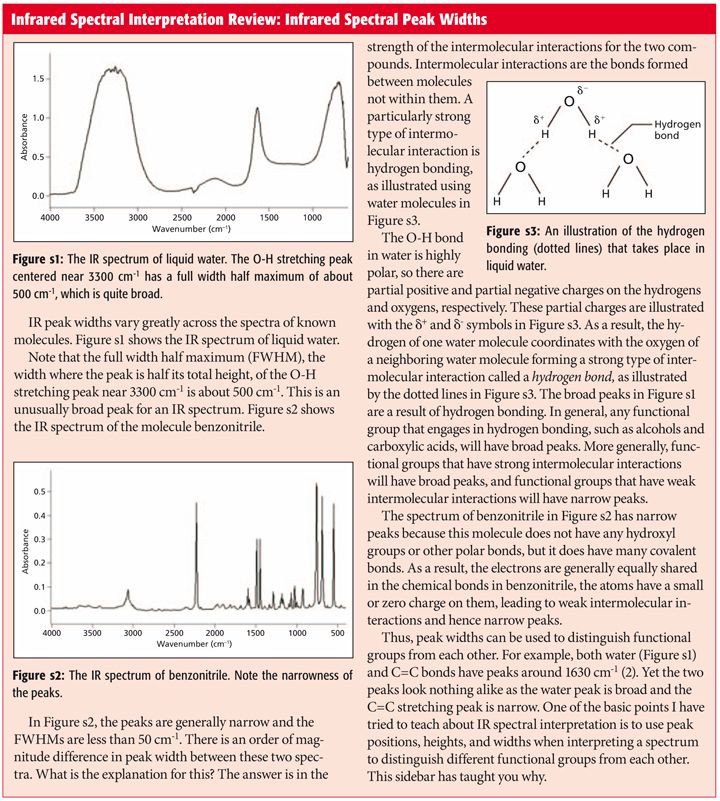
The C O Bond Part Iii Carboxylic Acids

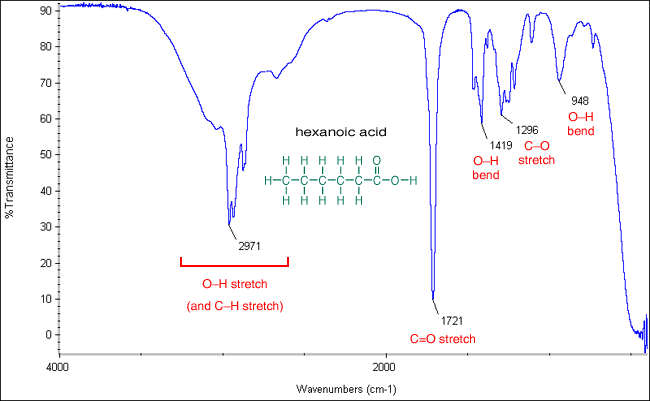
Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

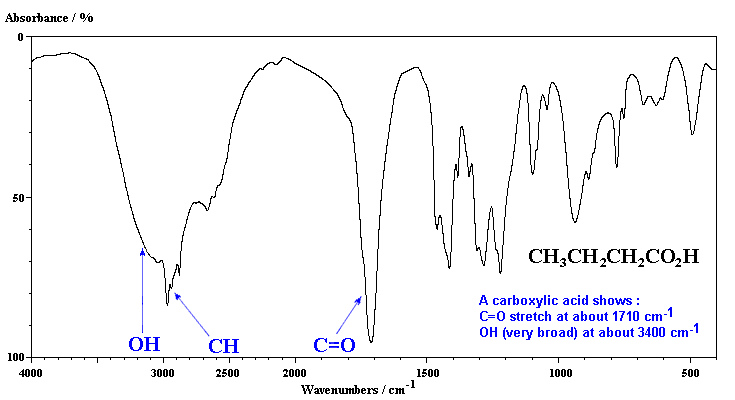
Infrared Spectrum Of Butanoic Acid C4h8o2 Ch3ch2ch2cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Butyric Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
The C O Bond Part Iii Carboxylic Acids

Interpretation Of Carboxylic Acid Ir Spectra Youtube

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate
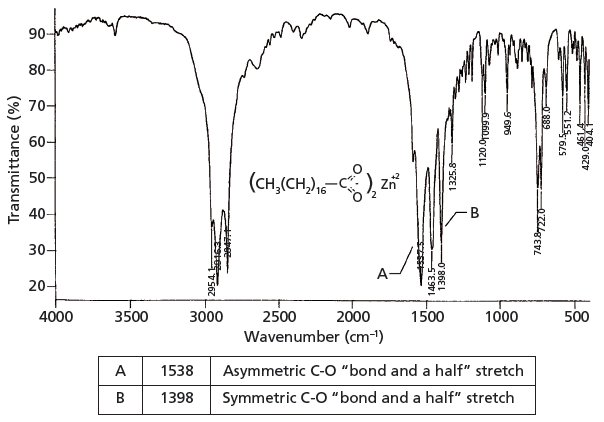
The Carbonyl Group Part V Carboxylates Coming Clean
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Lec15 Ir Spectra Of Carboxylic Acids Amides And Esters Youtube
Ir Infrared Absorption Bands Of Carboxylate